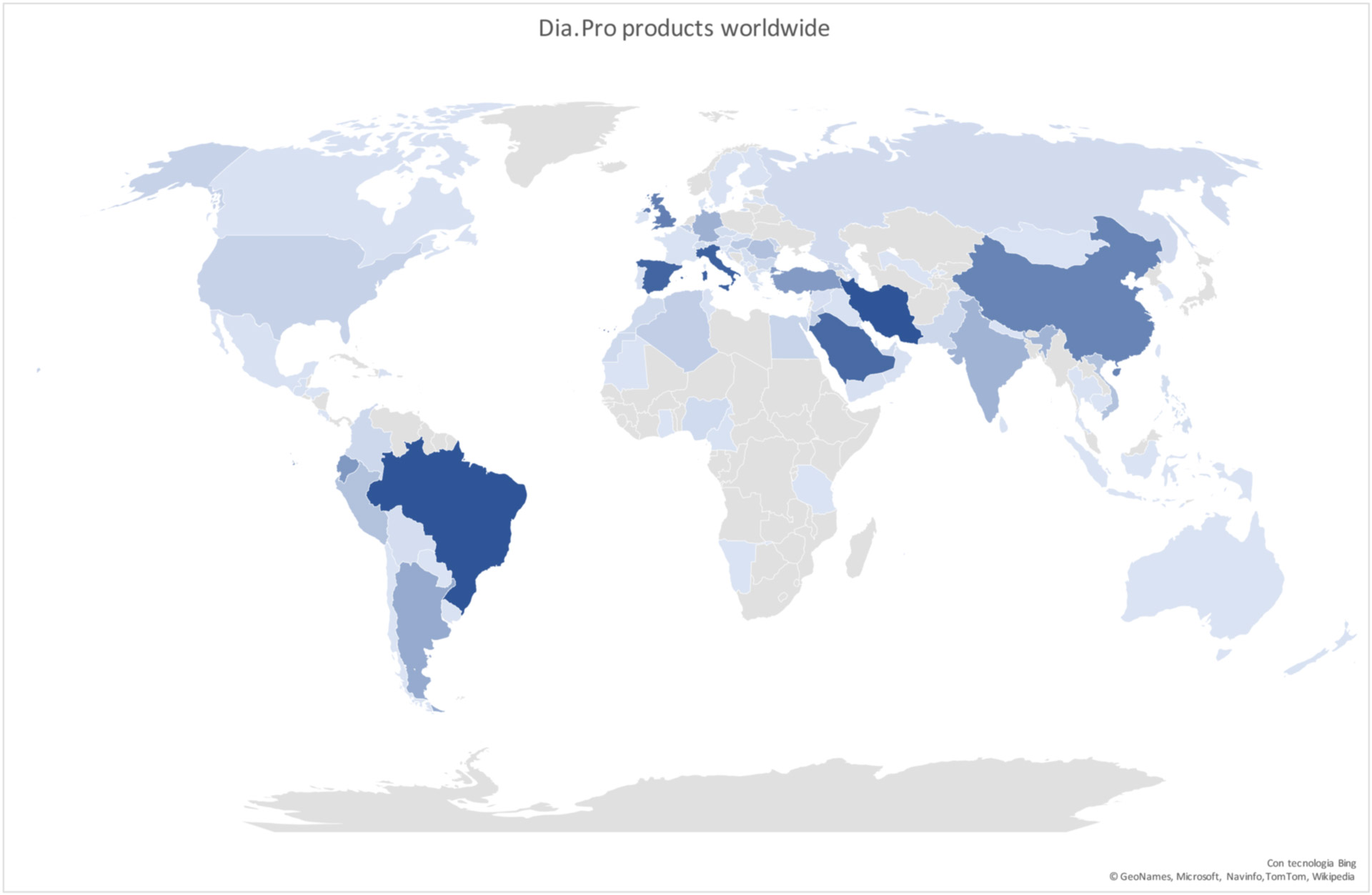
We offer an OEM production of CE marked DiaPro products/Immunoassays, through individual contracts of supply, providing the customer access DiaPro’s CE Design Dossiers & certification, to speed up the process of acquiring their own CE mark.
Development Projects for Diagnostic systems, by providing skilled & professional partnership &/or R&D contracts in assay development, performance evaluation trials and CE-marking of the assays.
Partnership in development Projects of new straight forward diagnostic devices, based on innovative technologies & new strategic reagents.
Technology Transfer dedicated to companies interested in extending their product range, on new devices or manufacturing in emerging countries.